Research
Program Areas
Chemical Synthesis Platform
Positron emission tomography (PET) is a highly sensitive whole-body imaging technique based on the detection of radiation from the decay of a radioactive probe ("tracer") administered to the patient. Unlike other types of imaging which primarily image the structure of the body, PET can sensitively measure the rate of particular biochemical processes including metabolic activity, cell signaling, DNA replication, cell proliferation, gene expression, etc., depending on the particular tracer administered. In the clinic, PET is used to diagnose, locate, and stage cancer, monitor the effectiveness of chemotherapy, and perform diagnostic bone scans. It can also be used to diagnose heart and mental health disorders, track the progression of drugs or toxins through the body, and monitor infectious disease processes. PET is also used extensively in disease research and drug development.
Despite the usefulness and potential of PET, research is hindered by the inability to acquire most tracers at reasonable cost. With current technologies, cost of production soars due to the need for specialized radiation safety infrastructure (e.g., hot cells), automated synthesis equipment, analytical testing equipment, and personnel trained in radiochemistry. A couple of tracers are available at an affordable price from commercial radiopharmacies, who divide the production costs over many customers, but thousands of other tracers remain largely unavailable.
The Chemical Synthesis Platform Technology program is focused on eliminating this roadblock, by developing systems to simplify, miniaturize and lower the cost of production of diverse PET tracers. Ultimately we aim to develop a standalone, user-friendly benchtop system that can synthesize a variety of radiotracers on demand. Systems in development span the entire process of PET tracer production including dispensing of the radioisotope, synthesis, purification and formulation of the tracer, and analytical testing of the final batch. Most of these systems incorporate microfluidic components to take advantage of the many scientific and practical benefits of working at small scales. We are also interested in developing tools that enable radiochemists to more easily and rapidly discover new tracers and optimize their synthesis.
Recent and current projects include: (i) a radioisotope and probe dispenser, (ii) a new “unit operations” based paradigm for developing automated synthesis programs, (iii) a 3-reactor high-pressure compatible synthesizer (ELIXYS) for synthesis of complex and diverse tracers, (iv) a chip-based microfluidic synthesizer, (v) a microfluidic system for optimizing reaction conditions for immunoPET, (vi) a microfluidic PET probe concentrator, and (vii) a Cerenkov imaging system to monitor processes within microfluidic chips.
Systems Biology
Systems biology is fundamental to the Crump Institute's goals to develop new in vitro and in vivo (imaging) molecular diagnostic technologies. By studying cells and organisms from a systemwide perspective, we learn what are the most informative events to detect and image to assess the healthy or diseased state of the patient's tissue. The Systems Biology program employs an integrated experimental and theoretical approach to study cancer and immune diseases, and to understand the interactions of microorganisms with the human host. Microbes form an intricate symbiotic system with the host. By studying the genomes and the transcriptomes of the microbes living inside humans, we aim to understand how microorganisms interact with and influence the human immune system and play a role in human health and disease.
Model systems, such as normal and cancer cells in culture, and small animal models of disease, provide controlled environments where the genetic composition and surrounding conditions can be defined and analyzed in a systematic fashion. Equally important is the ability to obtain serum and tissue from patients, that directly reflect the biology of human disease. From these biological samples, large-scale systemwide measurements can be made, of gene and protein expression that produce cell communication systems and metabolic activity to carry out normal functions of our organ systems and altered ones of disease. Finally, theoretical and computational approaches are used to untangle the complex network of interactions and feedback loops within cells and tissues. Our fundamental goal is to decipher the integrated circuits within and between cells - the networks that form the computational logic, or “thinking” of the cell that determines how it will react under normal conditions, or mis-react in disease.
The Systems Biology program works collaboratively with physicians and biologists studying diseases like cancer and immune disorders to help define what molecular events need to be detected or imaged based on the biology of the affected cells and tissues, and with the technology programs of the Crump to develop new methods to make experimental and diagnostic measurements.
In Vitro Molecular Diagnostics
IVMD
Circulating tumor cells (CTCs) are cancer cells that break away from either a primary tumor or metastatic site, and circulate in the peripheral blood as the cellular origin of metastasis. With their role as “tumor liquid biopsy”, CTCs provide convenient access to all disease sites, including that of the primary tumor and the site of fatal metastases. It is conceivable that detecting and analyzing CTCs will provide insightful information in assessing the disease status without the flaws and limitations encountered in performing conventional tumor biopsies. However, identifying CTCs in patient blood samples is technically challenging due to the extremely low abundance of CTCs among a large number of hematologic cells. To address this unmet need, there have been significant research endeavors devoted to developing CTC detection, isolation, and characterization technologies.
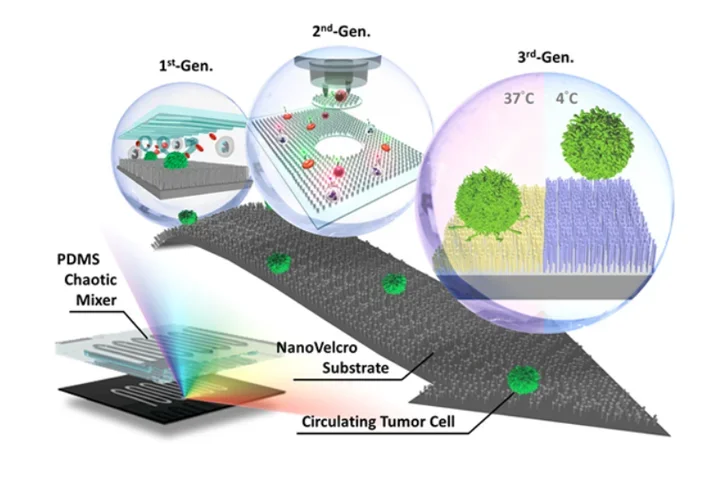
Inspired by the nanoscale interactions observed in the tissue microenvironment, our research team at UCLA pioneered a unique concept of “NanoVelcro” cell-affinity substrates, in which CTC capture agent-coated nanostructured substrates were utilized to immobilize CTCs with high efficiency. The working mechanism of NanoVelcro cell-affinity substrates mimics that of VelcroTM – when the two fabric strips of a Velcro fastener are pressed together, tangling between the hairy surfaces on two strips leads to strong binding. Through continuous evolution, 3 generations (gens) of NanoVelcro CTC Chips have been established to achieve different clinical utilities. The 1st-gen NanoVelcro Chip, composed of a silicon nanowire substrate (SiNS) and an overlaid microfluidic chaotic mixer, was created for CTC enumeration. Side-by-side analytical validation studies using clinical blood samples suggested that the sensitivity of 1st-gen NanoVelcro Chip outperforms that of FDA-approved CellSearchTM. In conjunction with the use of laser microdissection (LMD) technique, 2nd-gen NanoVelcro Chips (i.e., NanoVelcro-LMD), based on polymer nanosubstrates, were developed for single-CTC isolation.
The individually isolated CTCs can be subjected to single-CTC genotyping (e.g., Sanger sequencing and next-generation sequencing, NGS) to verify CTC’s role as tumor liquid biopsy. By grafting thermoresponsive polymer brushes onto SiNS, 3rd-gen NanoVelcro Chips (i.e., Thermoresponsive NanoVelcro) have demonstrated the capture and release of CTCs at 37 and 4°C, respectively. The temperature-dependent conformational changes of polymer brushes can effectively alter the accessibility of the capture agent on SiNS, allowing for rapid CTC purification with desired viability and molecular integrity. We envision that NanoVelcro CTC Assays will lead the way for powerful and cost-efficient diagnostic platforms for researchers to better understand underlying disease mechanisms and for physicians to monitor real-time disease progression.
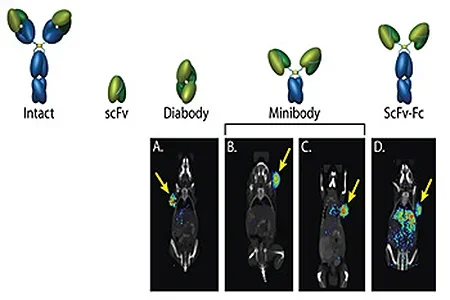
Molecular Imaging w/ImmunoPET
As part of the Crump Institute's broad goal of providing platform imaging strategies for detection of any molecular biomarker of choice, we are developing engineered antibodies as a means for imaging cell surface proteins in vivo. Approximately 20% of a cell's proteins are expressed on its surface, making them accessible to antibody-based molecular imaging probes. These cell surface biomarkers include important classes of proteins such as growth factor receptors, adhesion molecules, enzymes and proteases, tissue-specific markers, differentiation and activation markers — making the cell surface highly rich in informative targets that can reveal the biological state of the cell, both in normal states and in their transitions to disease.
Antibodies of high specificity for a selected protein target can be readily isolated by methods such as phage display. The ImmunoPET group then genetically engineers these antibodies for optimal function as imaging agents - rapid, high-level targeting to specific protein and low non-specific background in surrounding tissue. The ImmunoPET team relies on collaborations with the Systems Biology and Molecular Diagnostics programs to identify informative cell-surface proteins as biomarkers in disease, particularly in cancer and immune disorders. Implementation of these strategies requires close collaboration with the Chemical Synthesis and Preclinical Imaging programs to rapidly radiolabel and evaluate these novel tracers in animal models of disease.
Preclinical Imaging Systems
Preclinical imaging at the Crump Institute spans domains from in vitro diagnostics and imaging cell cultures, through in vivo imaging of mouse models of disease, using microPET, microCT, and optical imaging modalities. Overall, the focus of the Preclinical Imaging Systems program is to develop innovative technologies that enable the visualization and measurement of biological processes to facilitate research on the molecular mechanisms of cells in health and disease.
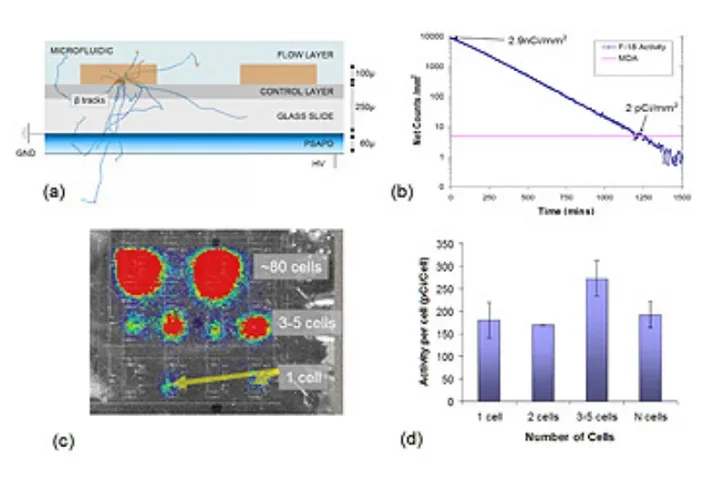
To further these goals, highly sensitive silicon-based solid state imaging sensors (Position Sensitive Avalanche Photodiode Detectors, or PSAPDs), are being developed that can detect radiolabeled probes at amounts as low as a few picocuries. These devices are integrated with microfluidic chips in collaborations with the in vitro Molecular Diagnostics and Chemical Synthesis Platform program areas to produce novel imaging devices for in vitro assays in arrays of cell cultures in chips.
For in vivo imaging, multimodality instrumentation is being designed and optimized for combining PET with anatomical imaging (such as CT) and optical imaging of mouse models of disease. These state-of-the-art technologies are made available to Crump investigators in coordination with the Preclinical Imaging Technology Center.